Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Page 1 of 2
Page 1 of 2 • 1, 2 
 Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
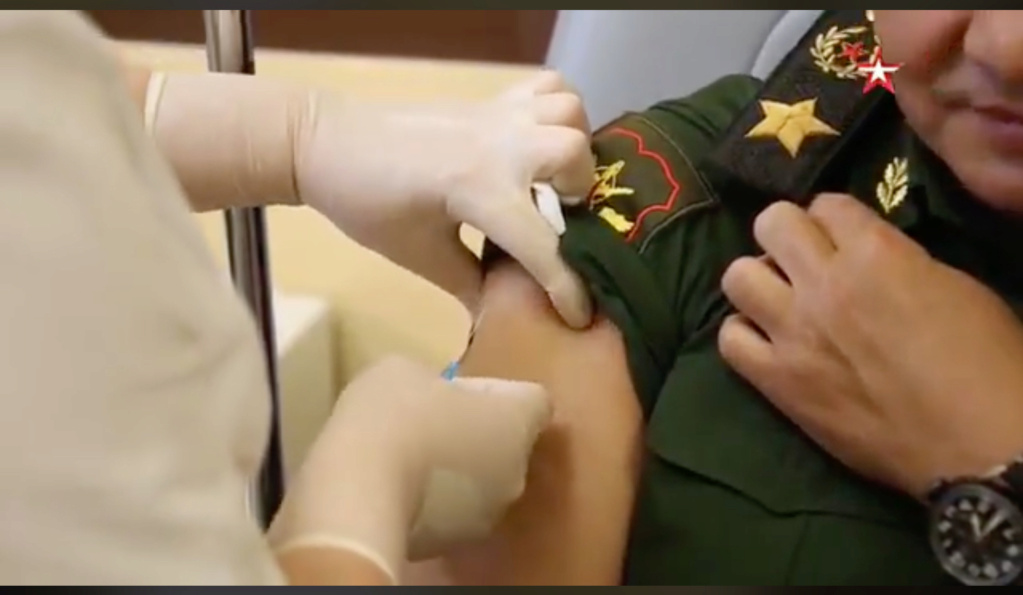
Rusko cjepivo protiv covida-19, Sputnik-V, izazvalo je stvaranje antitijela kod svih sudionika ranih faza kliničkih ispitivanja, pokazali su rezultati ispitivanja, objavljeni u petak u medicinskom časopisu The Lancet, što je Moskva pozdravila
Rusko cjepivo protiv Covid-19, Sputnik-V, izazvalo je stvaranje antitijela kod svih sudionika ranih faza kliničkih ispitivanja, pokazali su rezultati ispitivanja, objavljeni u petak u medicinskom časopisu The Lancet, što je Moskva pozdravila.
Rezultati dvaju ispitivanja, provedenih u lipnju i srpnju ove godine na ukupno 76 sudionika, pokazali su da je 100 posto sudionika razvilo antitijela na novi koronavirus i da nisu imali ozbiljnih nuspojava, navodi list. Rusija je registrirala cjepivo u dvije faze za domaću uporabu u kolovozu, prva na svijetu, i to prije objave bilo kakvih podataka o njemu i prije početka masovnog ispitivanja. „Dva ispitivanja u trajanju od 42 dana, koja su svako obuhvatila 38 zdravih odraslih ispitanika, nisu pokazala nikakve ozbiljne štetne posljedice među sudionicima, i potvrdila su da su kandidati za cjepiva izazvali stvaranje antitijela”, navodi The Lancet. „Velika, dugotrajna ispitivanja uključujući usporedbu s placebo skupinom, i daljnje praćenje potrebni su kako bi se utvrdila dugoročna sigurnost i učinkovitost cjepiva za sprječavanje zaraze Covidom-19”, dodaje se u članku.
Moskva pozdravlja rezultate ispitivanja „Ovime odgovaramo na sva pitanja Zapada koja su uredno postavljena u zadnja tri tjedna, iskreno, s očitim ciljem bacanja mrlje na rusko cjepivo”, rekao je Kirill Dmitriev, direktor Ruskog fonda za izravna ulaganja, državnog investicijskog fonda koji je financirao razvoj cjepiva. „Sada ćemo mi početi postavljati pitanja o nekim cjepivima sa Zapada”. Prvi rezultati masovnog ispitivanja ruskog cjepiva, koje je počelo prošli tjedan, očekuju se u listopadu ili studenom. Obuhvatit će 40.000 ljudi, a već se javilo 3000.
omentirajući rezultate prvih ispitivanja, glavni autor članka Naor Bar-Zeev, iz Međunarodnog centra za pristup cjepivima pri Bloombergovoj školi za javno zdravlje na Sveučilištu Johns Hopkins, rekao je da su ispitivanja „ohrabrujuća, ali malena”. Bar-Zeev je istaknuo da „još nije dokazana klinička učinkovitost nijednog cjepiva protiv covida-19”.
Pročitajte više na: https://www.vecernji.hr/vijesti/potvrdilo-ispitivanje-rusko-cjepivo-protiv-koronavirusa-izazvalo-stvaranje-antitijela-1428973 - www.vecernji.hr
.....................
Kreće čipiranje
Rusko cjepivo protiv Covid-19, Sputnik-V, izazvalo je stvaranje antitijela kod svih sudionika ranih faza kliničkih ispitivanja, pokazali su rezultati ispitivanja, objavljeni u petak u medicinskom časopisu The Lancet, što je Moskva pozdravila.
Rezultati dvaju ispitivanja, provedenih u lipnju i srpnju ove godine na ukupno 76 sudionika, pokazali su da je 100 posto sudionika razvilo antitijela na novi koronavirus i da nisu imali ozbiljnih nuspojava, navodi list. Rusija je registrirala cjepivo u dvije faze za domaću uporabu u kolovozu, prva na svijetu, i to prije objave bilo kakvih podataka o njemu i prije početka masovnog ispitivanja. „Dva ispitivanja u trajanju od 42 dana, koja su svako obuhvatila 38 zdravih odraslih ispitanika, nisu pokazala nikakve ozbiljne štetne posljedice među sudionicima, i potvrdila su da su kandidati za cjepiva izazvali stvaranje antitijela”, navodi The Lancet. „Velika, dugotrajna ispitivanja uključujući usporedbu s placebo skupinom, i daljnje praćenje potrebni su kako bi se utvrdila dugoročna sigurnost i učinkovitost cjepiva za sprječavanje zaraze Covidom-19”, dodaje se u članku.
Moskva pozdravlja rezultate ispitivanja „Ovime odgovaramo na sva pitanja Zapada koja su uredno postavljena u zadnja tri tjedna, iskreno, s očitim ciljem bacanja mrlje na rusko cjepivo”, rekao je Kirill Dmitriev, direktor Ruskog fonda za izravna ulaganja, državnog investicijskog fonda koji je financirao razvoj cjepiva. „Sada ćemo mi početi postavljati pitanja o nekim cjepivima sa Zapada”. Prvi rezultati masovnog ispitivanja ruskog cjepiva, koje je počelo prošli tjedan, očekuju se u listopadu ili studenom. Obuhvatit će 40.000 ljudi, a već se javilo 3000.
omentirajući rezultate prvih ispitivanja, glavni autor članka Naor Bar-Zeev, iz Međunarodnog centra za pristup cjepivima pri Bloombergovoj školi za javno zdravlje na Sveučilištu Johns Hopkins, rekao je da su ispitivanja „ohrabrujuća, ali malena”. Bar-Zeev je istaknuo da „još nije dokazana klinička učinkovitost nijednog cjepiva protiv covida-19”.
Pročitajte više na: https://www.vecernji.hr/vijesti/potvrdilo-ispitivanje-rusko-cjepivo-protiv-koronavirusa-izazvalo-stvaranje-antitijela-1428973 - www.vecernji.hr
.....................
Kreće čipiranje

epikur37- Posts : 45339
2015-08-06

Hektorović- Posts : 26373
2018-04-10
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
[size=39] [/size]
[/size]
[size=39]IZNENAĐENJE?[/size]
[size=87]Rezultati ispitivanja ruskog cjepiva objavljeni u uglednom časopisu: ‘Svi su razvili antitijela!‘[/size]
https://www.jutarnji.hr/vijesti/svijet/rezultati-ispitivanja-ruskog-cjepiva-objavljeni-u-uglednom-casopisu-svi-su-razvili-antitijela-15017161
 [/size]
[/size][size=39]IZNENAĐENJE?[/size]
[size=87]Rezultati ispitivanja ruskog cjepiva objavljeni u uglednom časopisu: ‘Svi su razvili antitijela!‘[/size]
https://www.jutarnji.hr/vijesti/svijet/rezultati-ispitivanja-ruskog-cjepiva-objavljeni-u-uglednom-casopisu-svi-su-razvili-antitijela-15017161

epikur37- Posts : 45339
2015-08-06
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Nema ništa od toga. Nevalja. Morate kupovat američko.

RayMabus- Posts : 184105
2014-04-11
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Eto dočekat ćemo i da barba ero pruži rukicu kako bi ga spasila ruska tehnologija :)

epikur37- Posts : 45339
2015-08-06

Hektorović- Posts : 26373
2018-04-10
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
sve jedno ne cijepim se od bolesti koju je u RH prema serološkim testovima preboljelo preko 200 000 ljudi, a samo je jedna osoba mlađa od 50godina (47godina) umrla(a imala je hrpu bolestiju).
dakle šansa da umrem je manja od 1:200 000 , super što imaju cijepivo.. sa njime cijepiti sve starije od 60godina, te ekipu u bolnicam i doktore i sestre.. i od mlađih tko hoće dobrovoljno
dakle šansa da umrem je manja od 1:200 000 , super što imaju cijepivo.. sa njime cijepiti sve starije od 60godina, te ekipu u bolnicam i doktore i sestre.. i od mlađih tko hoće dobrovoljno
_________________
May Allah destroy Australia

AssadNaPodmornici- Posts : 22267
2018-06-14
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
AssadNaPodmornici wrote:sve jedno ne cijepim se od bolesti koju je u RH prema serološkim testovima preboljelo preko 200 000 ljudi, a samo je jedna osoba mlađa od 50godina (47godina) umrla(a imala je hrpu bolestiju).
dakle šansa da umrem je manja od 1:200 000 , super što imaju cijepivo.. sa njime cijepiti sve starije od 60godina, te ekipu u bolnicam i doktore i sestre.. i od mlađih tko hoće dobrovoljno
Studente zbog profesora

Hektorović- Posts : 26373
2018-04-10
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Cijepljenje će biti obvezno.

epikur37- Posts : 45339
2015-08-06
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
epikur37 wrote:Cijepljenje će biti obvezno.
Ruje su krenule već sad, Ameri misle za mjesec... Vidim neki bi kod nas čekali još do sredine sljedeće godine, no mislim kako će ekonomski pritisak sve to ubrzati.

Hektorović- Posts : 26373
2018-04-10

Hektorović- Posts : 26373
2018-04-10
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
jel to znaci da se Gates nece morat vise sekirat
zabrino se bio opasno
zabrino se bio opasno
_________________
It was always the women, and above all the young ones, who were the most bigotet adherents of the party, the swallowers of slogans, the amateur spies and nosers-out of unortodoxy.
Orwell 1984

prckov- Posts : 34555
2014-04-19
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
ko zeli nek izvoli
nek se cepa
uopste ne kapiram
to napinjanje da se
dokaze da je ruska vakcina ok
najbolja je, hvala, ne !
nek se cepa
uopste ne kapiram
to napinjanje da se
dokaze da je ruska vakcina ok
najbolja je, hvala, ne !
_________________
ne znam ko ce da pobedi
ali znam ko nece da izgubi !

pizzon- Posts : 5017
2020-04-21
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
epikur37 wrote:Eto dočekat ćemo i da barba ero pruži rukicu kako bi ga spasila ruska tehnologija :)

garanutujem, neces biti u prilici.
ja se nadam da cu imati alternativu.
za lovu.

pizzon- Posts : 5017
2020-04-21
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Upravo ga tješe... Bile ima još virusa, izaći će štagot opet :))prckov wrote:jel to znaci da se Gates nece morat vise sekirat
zabrino se bio opasno
_________________
Iduća dva tjedna su ključna

mutava baštarda- Posts : 21037
2015-09-14
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
_________________
Iduća dva tjedna su ključna

mutava baštarda- Posts : 21037
2015-09-14
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
al obrati paznju, jutarnji objavi da su razvili imunitet al nije dokazana klinicka ucinkovitostepikur37 wrote:[size=39][/size]
[size=39]IZNENAĐENJE?[/size]
[size=87]Rezultati ispitivanja ruskog cjepiva objavljeni u uglednom časopisu: ‘Svi su razvili antitijela!‘[/size]
https://www.jutarnji.hr/vijesti/svijet/rezultati-ispitivanja-ruskog-cjepiva-objavljeni-u-uglednom-casopisu-svi-su-razvili-antitijela-15017161
legende su
mislim da im no cnn nije ravan
_________________
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
nije jutarnji ništa objavio, jutarnji je prenio objavu,Leviathan2 wrote:al obrati paznju, jutarnji objavi da su razvili imunitet al nije dokazana klinicka ucinkovitostepikur37 wrote:[size=39][/size]
[size=39]IZNENAĐENJE?[/size]
[size=87]Rezultati ispitivanja ruskog cjepiva objavljeni u uglednom časopisu: ‘Svi su razvili antitijela!‘[/size]
https://www.jutarnji.hr/vijesti/svijet/rezultati-ispitivanja-ruskog-cjepiva-objavljeni-u-uglednom-casopisu-svi-su-razvili-antitijela-15017161
legende su
mislim da im no cnn nije ravan
ovo je izvorni tekst...In The Lancet, Denis Y Logunov and colleagues from the N F Gamaleya Research Institute of Epidemiology and Microbiology in Russia present findings from two phase 1/2, non-randomised, open-label studies of a heterologous, replication-deficient, recombinant adenovirus vector-based vaccine in both frozen and lyophilised formulations.
1
The researchers enrolled 76 healthy adult volunteers (aged 18–60 years) into the two studies (38 people in each study); 53 (70%) participants were men and 23 (30%) were women. The primary outcome measures of the studies were safety and immunogenicity (antigen-specific humoral immunity). In phase 1 of each three-arm study, two groups of nine volunteers received one dose of either recombinant adenovirus type 26 (rAd26) vector or recombinant adenovirus type 5 (rAd5) vector, both carrying the gene for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein (rAd26-S and rAd5-S), in lyophilised or frozen form. In phase 2, another group of 20 healthy adult volunteers in each study received sequential doses of rAd26-S followed by rAd5-S of one of the two formulations. Adverse events were mostly mild, with the most common adverse events being pain at injection site (44 [58%]), hyperthermia (38 [50%]), headache (32 [42%]), asthenia (21 [28%]), and muscle and joint pain (18 [24%]). Adverse events occurred at similar frequency for each vaccine vector, with each formulation, and after each dose. Serious adverse events did not arise in this small cohort. Both formulations of the vaccine were immunogenic in all participants, inducing neutralising humoral and cell-mediated responses. In phase 2, 85% of participants had detectable antibodies at 14 days after the priming dose, rising to 100% by day 21, with substantial titre rises after the boosting dose.
The two studies by Logunov and colleagues have several strengths. First, adenoviruses are ubiquitous, so humans might not be immunologically naive. Previous immunity to the vector might interfere with adenovirus-vectored COVID-19 vaccine efficacy. Indeed, findings of an adenovirus type 5-vectored COVID-19 vaccine trial suggested such immunity could affect COVID-19 responses.
2
In another study, a chimpanzee adenovirus vector was used, since humans will presumably be naive to at least the first dose.
3
Logunov and colleagues used immunologically distinct (heterologous) vectors in their two-dose (prime-boost) regimen. They investigated cross-vector heterologous immunity and previous antivector adenovirus immunity, and neither affected COVID-19 immunogenicity.
A second strength is the threshold for neutralisation used in the two studies. Neutralisation assays vary from study to study. Neutralisation is tested by examining whether plasma from a recently vaccinated individual can prevent cellular damage on in-vitro exposure of cells to SARS-CoV-2. Both the degree of such protection (how many damaged cells are allowed) and the dose of the infecting virus vary across studies. Expecting a vaccine to result in less than complete neutralisation is not inherently wrong but sets the bar low and makes it easier to claim neutralising activity. In Logunov and colleagues’ studies, however, the threshold for neutralisation was set high in two regards: the inoculating viral dose was large, and no arising cellular damage was allowable. Essentially, the assay was set at full neutralisation. This high bar implies these researchers took an a-priori risk that their vaccine might fail the test. It did not. It remains to be seen if other manufacturers will set a similar high standard.
A third strength is that the vaccine, similar to other adenovirus-vectored and mRNA COVID-19 vaccines before it,
2
,
3
,
4
induced broad immune responses. Although not specifically discussed, the results imply a T-helper-1-cell-weighted response that might be important for vaccine safety, potentially reducing the risk of antibody-dependent enhanced disease.
5
A fourth strength was development of two vaccine formulations, frozen and lyophilised. A lyophilised formulation could mean stability within the existing global vaccine refrigerated cold chain that is needed to maintain vaccine efficacy from factory to recipient, a hurdle other vaccines are yet to address. Although more costly to produce at scale, product stability will maximise reach in remote terrain, a must if universal and equitable coverage is to be achieved.
Some limitations of the studies by Logunov and colleagues are notable. In the study of the frozen vaccine formulation, the population included young military personnel. Soldiers are likely to be fitter and healthier than the general population. Moreover, in older adults, immune senescence might make vaccines less immunogenic, and this age group was absent from this study. Sex imbalance occurred in the study arms because there was no random allocation. A control arm was conspicuously absent. Two participants were of Asian descent, with the rest of the participants of white European ethnic origin. Clearly, much more remains to be learned from the phase 3 randomised trial planned to include 40 000 civilian volunteers and, hopefully, broadly inclusive of groups at risk.
This COVID-19 vaccine candidate from Russia joins two other adenovirus-vectored COVID-19 vaccine candidates, which have been reported in randomised trials in The Lancet,
2
,
3
and an mRNA vaccine candidate reported in a non-randomised trial.
4
Similar to these studies before it, Logunov and colleagues’ studies are encouraging but small. The immunogenicity bodes well, although nothing can be inferred on immunogenicity in older age groups, and clinical efficacy for any COVID-19 vaccine has not yet been shown. A wide portfolio of early COVID-19 vaccine candidates will hopefully provide more successful vaccines that are broadly protective across risk groups and that increase the global availability of what will be a precious limited commodity.
Showing safety will be crucial with COVID-19 vaccines, not only for vaccine acceptance but also for trust in vaccination broadly.
6
Safety outcomes up to now are reassuring, but studies to date are too small to address less common or rare serious adverse events. Unlike clinical trials of therapeutics, in which safety is balanced against benefit in patients, vaccine trials have to balance safety against infection risk, not against disease outcome. Since vaccines are given to healthy people and, during the COVID-19 pandemic, potentially to everyone after approval following phase 3 trials, safety is paramount. Licensure in most settings should depend on proven short-term and long-term efficacy against disease (not just immunogenicity) and more complete safety data, including rates of vaccine failure to prevent infection (not merely disease) and rates of occurrence of antibody-dependent enhanced disease, ideally from very large scale, flexible multivaccine trials with prolonged, blinded safety and efficacy follow-up.
6
,
7
Safety assurance will then require further large-scale surveillance after licensure. Such surveillance is not well established in many settings, and rapid efforts need to be made by governments, regulators, and global research funders to get those systems in place. Surveillance will also be vital for showing transmission reduction, which is unlikely to come from phase 3 trials since these are powered to detect COVID-19 disease outcomes and not asymptomatic SARS-CoV-2 infection. To be sure, most past vaccines were designed to target disease and not infection as such, but with COVID-19, the general public could be expecting striking reductions in disease transmission after widespread vaccine introduction. Such effects would be very welcome if they occur, but they are far from certain. A vaccine that reduces disease but does not prevent infection might paradoxically make things worse. It could falsely reassure recipients of personal invulnerability, thus reducing transmission-mitigating behaviours. In turn, this could lead to increased exposure among older adults in whom efficacy is likely to be lower, or among other higher-risk groups who might have lower vaccine acceptance and uptake. In view of the ongoing painful toll of the COVID-19 pandemic and its magnitude, the more vaccine candidates that have successful early results the better. Ultimately, all vaccine candidates will need to show safety and prove durable clinical efficacy (including in groups at greater risk) in large randomised trials before they can be put into widespread use. Equitable access will require multiple vaccine producers and providers in a range of settings. Each of their successes will together lead us towards our collective, longed for, new day.

michaellcmacha- Posts : 21325
2015-08-08
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
ovo sto je jutarnji objavio nema blage veze s tim sto si ti postaomichaellcmacha wrote:nije jutarnji ništa objavio, jutarnji je prenio objavu,Leviathan2 wrote:al obrati paznju, jutarnji objavi da su razvili imunitet al nije dokazana klinicka ucinkovitostepikur37 wrote:[size=39][/size]
[size=39]IZNENAĐENJE?[/size]
[size=87]Rezultati ispitivanja ruskog cjepiva objavljeni u uglednom časopisu: ‘Svi su razvili antitijela!‘[/size]
https://www.jutarnji.hr/vijesti/svijet/rezultati-ispitivanja-ruskog-cjepiva-objavljeni-u-uglednom-casopisu-svi-su-razvili-antitijela-15017161
legende su
mislim da im no cnn nije ravan
ovo je izvorni tekst...In The Lancet, Denis Y Logunov and colleagues from the N F Gamaleya Research Institute of Epidemiology and Microbiology in Russia present findings from two phase 1/2, non-randomised, open-label studies of a heterologous, replication-deficient, recombinant adenovirus vector-based vaccine in both frozen and lyophilised formulations.
1
The researchers enrolled 76 healthy adult volunteers (aged 18–60 years) into the two studies (38 people in each study); 53 (70%) participants were men and 23 (30%) were women. The primary outcome measures of the studies were safety and immunogenicity (antigen-specific humoral immunity). In phase 1 of each three-arm study, two groups of nine volunteers received one dose of either recombinant adenovirus type 26 (rAd26) vector or recombinant adenovirus type 5 (rAd5) vector, both carrying the gene for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein (rAd26-S and rAd5-S), in lyophilised or frozen form. In phase 2, another group of 20 healthy adult volunteers in each study received sequential doses of rAd26-S followed by rAd5-S of one of the two formulations. Adverse events were mostly mild, with the most common adverse events being pain at injection site (44 [58%]), hyperthermia (38 [50%]), headache (32 [42%]), asthenia (21 [28%]), and muscle and joint pain (18 [24%]). Adverse events occurred at similar frequency for each vaccine vector, with each formulation, and after each dose. Serious adverse events did not arise in this small cohort. Both formulations of the vaccine were immunogenic in all participants, inducing neutralising humoral and cell-mediated responses. In phase 2, 85% of participants had detectable antibodies at 14 days after the priming dose, rising to 100% by day 21, with substantial titre rises after the boosting dose.
The two studies by Logunov and colleagues have several strengths. First, adenoviruses are ubiquitous, so humans might not be immunologically naive. Previous immunity to the vector might interfere with adenovirus-vectored COVID-19 vaccine efficacy. Indeed, findings of an adenovirus type 5-vectored COVID-19 vaccine trial suggested such immunity could affect COVID-19 responses.
2
In another study, a chimpanzee adenovirus vector was used, since humans will presumably be naive to at least the first dose.
3
Logunov and colleagues used immunologically distinct (heterologous) vectors in their two-dose (prime-boost) regimen. They investigated cross-vector heterologous immunity and previous antivector adenovirus immunity, and neither affected COVID-19 immunogenicity.
A second strength is the threshold for neutralisation used in the two studies. Neutralisation assays vary from study to study. Neutralisation is tested by examining whether plasma from a recently vaccinated individual can prevent cellular damage on in-vitro exposure of cells to SARS-CoV-2. Both the degree of such protection (how many damaged cells are allowed) and the dose of the infecting virus vary across studies. Expecting a vaccine to result in less than complete neutralisation is not inherently wrong but sets the bar low and makes it easier to claim neutralising activity. In Logunov and colleagues’ studies, however, the threshold for neutralisation was set high in two regards: the inoculating viral dose was large, and no arising cellular damage was allowable. Essentially, the assay was set at full neutralisation. This high bar implies these researchers took an a-priori risk that their vaccine might fail the test. It did not. It remains to be seen if other manufacturers will set a similar high standard.
A third strength is that the vaccine, similar to other adenovirus-vectored and mRNA COVID-19 vaccines before it,
2
,
3
,
4
induced broad immune responses. Although not specifically discussed, the results imply a T-helper-1-cell-weighted response that might be important for vaccine safety, potentially reducing the risk of antibody-dependent enhanced disease.
5
A fourth strength was development of two vaccine formulations, frozen and lyophilised. A lyophilised formulation could mean stability within the existing global vaccine refrigerated cold chain that is needed to maintain vaccine efficacy from factory to recipient, a hurdle other vaccines are yet to address. Although more costly to produce at scale, product stability will maximise reach in remote terrain, a must if universal and equitable coverage is to be achieved.
Some limitations of the studies by Logunov and colleagues are notable. In the study of the frozen vaccine formulation, the population included young military personnel. Soldiers are likely to be fitter and healthier than the general population. Moreover, in older adults, immune senescence might make vaccines less immunogenic, and this age group was absent from this study. Sex imbalance occurred in the study arms because there was no random allocation. A control arm was conspicuously absent. Two participants were of Asian descent, with the rest of the participants of white European ethnic origin. Clearly, much more remains to be learned from the phase 3 randomised trial planned to include 40 000 civilian volunteers and, hopefully, broadly inclusive of groups at risk.
This COVID-19 vaccine candidate from Russia joins two other adenovirus-vectored COVID-19 vaccine candidates, which have been reported in randomised trials in The Lancet,
2
,
3
and an mRNA vaccine candidate reported in a non-randomised trial.
4
Similar to these studies before it, Logunov and colleagues’ studies are encouraging but small. The immunogenicity bodes well, although nothing can be inferred on immunogenicity in older age groups, and clinical efficacy for any COVID-19 vaccine has not yet been shown. A wide portfolio of early COVID-19 vaccine candidates will hopefully provide more successful vaccines that are broadly protective across risk groups and that increase the global availability of what will be a precious limited commodity.
Showing safety will be crucial with COVID-19 vaccines, not only for vaccine acceptance but also for trust in vaccination broadly.
6
Safety outcomes up to now are reassuring, but studies to date are too small to address less common or rare serious adverse events. Unlike clinical trials of therapeutics, in which safety is balanced against benefit in patients, vaccine trials have to balance safety against infection risk, not against disease outcome. Since vaccines are given to healthy people and, during the COVID-19 pandemic, potentially to everyone after approval following phase 3 trials, safety is paramount. Licensure in most settings should depend on proven short-term and long-term efficacy against disease (not just immunogenicity) and more complete safety data, including rates of vaccine failure to prevent infection (not merely disease) and rates of occurrence of antibody-dependent enhanced disease, ideally from very large scale, flexible multivaccine trials with prolonged, blinded safety and efficacy follow-up.
6
,
7
Safety assurance will then require further large-scale surveillance after licensure. Such surveillance is not well established in many settings, and rapid efforts need to be made by governments, regulators, and global research funders to get those systems in place. Surveillance will also be vital for showing transmission reduction, which is unlikely to come from phase 3 trials since these are powered to detect COVID-19 disease outcomes and not asymptomatic SARS-CoV-2 infection. To be sure, most past vaccines were designed to target disease and not infection as such, but with COVID-19, the general public could be expecting striking reductions in disease transmission after widespread vaccine introduction. Such effects would be very welcome if they occur, but they are far from certain. A vaccine that reduces disease but does not prevent infection might paradoxically make things worse. It could falsely reassure recipients of personal invulnerability, thus reducing transmission-mitigating behaviours. In turn, this could lead to increased exposure among older adults in whom efficacy is likely to be lower, or among other higher-risk groups who might have lower vaccine acceptance and uptake. In view of the ongoing painful toll of the COVID-19 pandemic and its magnitude, the more vaccine candidates that have successful early results the better. Ultimately, all vaccine candidates will need to show safety and prove durable clinical efficacy (including in groups at greater risk) in large randomised trials before they can be put into widespread use. Equitable access will require multiple vaccine producers and providers in a range of settings. Each of their successes will together lead us towards our collective, longed for, new day.
to sto si ti postao je strucno objasnjenje, strucni clanak,
ovo sto je jutarnji napisao je kvazinovinarstvo
_________________
 Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Re: Potvrdilo ispitivanje: Rusko cjepivo izazvalo stvaranje antitijela
Ja bih pristao kad bi pisalo latinicom.

crvenkasti-
Posts : 29707
2014-04-17
Page 1 of 2 • 1, 2 
 Similar topics
Similar topics» Drugo rusko cjepivo registrirano
» Srbija će proizvoditi rusko cjepivo
» Rusko cjepivo radi bez greške
» Stiglo rusko cjepivo u Mađarsku
» Ursula viče na Hrvatsku jer želi rusko cjepivo
» Srbija će proizvoditi rusko cjepivo
» Rusko cjepivo radi bez greške
» Stiglo rusko cjepivo u Mađarsku
» Ursula viče na Hrvatsku jer želi rusko cjepivo
Page 1 of 2
Permissions in this forum:
You cannot reply to topics in this forum
 Events
Events Latest images
Latest images
 by epikur37 4/9/2020, 17:06
by epikur37 4/9/2020, 17:06